The Electro-Etching Unit: What you will need
• Vertical tank
• Power supply - Direct Current (DC)
• Cathode grid
• Electrolytes
Vertical Tank

Always use a plastic tank, never metal. You can begin with small plates and an improvised tank made from a plastic water container with its top cut off, until you become familiar with the technique. You can use a flat tank or tray too: Cedric Green provides information about this method on his GREEN PRINTS website.
DIY tank with a plastic water container
Power Supply

You will need a varible power source preferably with a voltage output which can be varied, that supplies direct current (DC) up to 3 to 5 amps at least with a voltage of about 5 volts, equipped with digital displays and power controls. This power supply will provide a constant current flow and more secure control over the process, especially for those who are beginners and have no knowledge of electrical etching. Cedric Green shows how you can prepare your own power supply or use batteries (cheaper systems). You can use whichever power supply you want, but always use direct current (DC).
Direct Current Power Supply
For those who wish to obtain uniform and controlled results, my recommendation is to buy a power supply (DC) with digital displays, voltage and current (amperage) controls. These can be found from an online electronics retailer*. These devices are very practical and provide a voltage of between 0 to 10 volts and a current of 16 amps. They can be obtained for about €350.
Cathode Grid
In order for the electrolytic process to take place, a metal plate must be immersed in the tank and connected to the negative pole (-) cathode, and positioned opposite (in parallel with) another plate of the same metal, which is connected to the positive pole (+) anode. The plate that is attached to the anode is the plate that will be etched.
To simplify the process, instead of a cathode plate, you can use a stainless steel grid that fits the dimensions of your tank. You can appropriate the type of stainless steel grill popularly used for roasting meat. I would recommend using a different grid or grill for each of the metals that you are intending to etch.
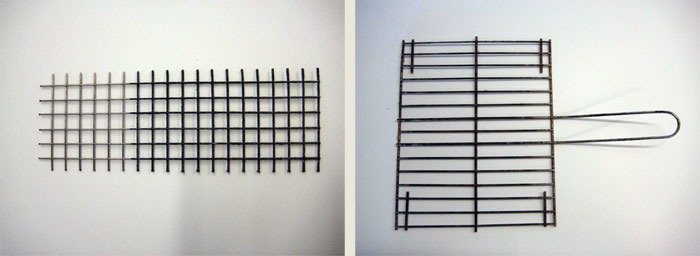
Stainless steel Cathode grid Stainless steel Cathode grill
Electrolyte
The electrically conductive salt solution is the electrolyte. It must have a cation (a positively charged ion) that is the same as the metal to be etched. Modern electro-etching experiences tell us that an electrolyte must generally use a salt dissolved in water containing positive ions that are the same as the metal to be etched.
So remember: You must make sure that the type of electrolyte you use is that of the same metal to be etched.
• COPPER SULFATE (Cu SO4) for COPPER plates
• ZINC SULFATE (Zn SO4) for ZINC plates
• FERROUS SULFATE (Fe SO4) for IRON and STEEL plates
These sulfates can be obtained from suppliers of industrial chemicals. I recommend using pure sulfate, free of any impurities that can cause unknown waste and undesirable consequences in disposal. If you buy sulfate in garden stores, be very careful that it has not been mixed with other products such as fungicides etc., as the resulting electrolyte may not work.
Because the electrolyte solutions are not exhausted with successive bites, and therefore they do not have to be continually renewed, I would advise using maximum purity sulfates.

Copper sulfate Zinc sulfate
Copper sulfate, zinc sulfate and ferrous sulfate are salts which are usually supplied in the form of crystals, or sometimes crushed into coarse grains. When handling these crystals or powders, you should avoid breathing in the dust or allowing the material to come into direct contact with the skin. Protect yourself with a dust mask and rubber gloves while they are solid salts. Once the salts are diluted in water there is no danger of breathing in the solutions. The solutions are safe because, as explained above, they do not emit harmful gases. However, you should avoid letting the electrolyte solutions touch the skin, and be careful when you are immersing or taking plates out of tanks so as not to cause splashing into the eyes. Use goggles to be extra safe. (See more about Precautions and Safety below).
The concentration (amount of salt per liter of water) of metal ions in the electrolytic solution will determine the current flow and, consequently, the amount of metal that will be dissolved from the plate you are etching. Low concentrations work slower but give effective etching. Saturated concentrations are faster but require a power supply with more current output. A high concentration combined with a high voltage can stimulate the generation of oxygen at the anode, forming - if the plate is copper - a thin but solid oxide layer, which is not dangerous but can cause etching to stop as the copper oxide is not electrically conductive. If this happens you must dilute the electrolyte.
If the plate is zinc, and the electrolytic solution is fairly concentrated, and used with a high voltage, the solution will slowly begin to change. As the zinc hydroxide is very alkaline it will increase the pH of the solution and thicken it. The earliest attempts at anodic etching were geared to these facts, and it necessitated the use of acids to neutralize the effects. There were etchers who added a small amount of sulfuric acid (0.03% / liter of solution) to their copper sulfate solution to prevent disagreeable oxidations - something which works on copper plates; but it is not recommended that sulfuric acid be added to zinc sulfate solutions since the current flow will produce a large quantity of gases.
I have worked with several electrolytic concentrations, each of them has given me slightly different nuances. I recommend you start experimenting with a low concentration in the solution. Later, if you have sufficient amperage of power supply, you can increase concentration and continue practicing and researching.
Electrolyte to etch COPPER plates
Concentration
Weak: 160g COPPER SULFATE to 1 liter of WATER
Medium: 200g COPPER SULFATE to 1 liter of WATER
Strong: 250g COPPER SULFATE to 1 liter of WATER
Electrolyte to etch ZINC plates:
Concentration
Weak: 160g ZINC SULFATE to 1 liter of WATER
Medium: 300g ZINC SULFATE to 1 liter of WATER
Strong: 500g ZINC SULFATE to 1 liter of WATER
Electrolyte to etch STEEL plates:
Concentration
Medium: 200g FERROUS SULFATE to 1 liter of WATER
Strong: 250g FERROUS SULFATE to 1 liter of WATER
• Electrolytic solutions should be made with neutral pH water, thus the chemistry of the electrolyte will be more balanced.
• Remember, you must use a wooden stick to dilute the salts in water, never metal.
• Before you fill the tank, prepare the electrolyte in advance, in a plastic container - you can filter it if necessary.
• Add one part sulfate salts to the water. Stir gently. Let the mixture stand a few minutes, then add more sulfate. Stir until the crystals are completely diluted.
• A concentration is measured as the amount of salt per liter of water.
• The temperature of the solution is very important. The electrolytic bath should be kept at BELOW 32º C to prevent the electrolyte penetrate between the metal and resist.
Electrolyte solutions that are not being used can be left in the vertical tank. Cover the tank to avoid the solution getting contaminated or evaporated in warm weather. If you are using small quantities, the solutions can be stored in plastic bottles with caps, with an information label indicating the concentration of sulfates, date, etc.
NOTE:
A solution of sodium chloride has been used by some etchers as an electrolyte. However, others have reservations about its use . A discussion of the issues can be found in the accompanying article "The basis of electro-etching a simplified explanation".
* Power supplies can be purchased from companies such as Multmeter Ware House or MED-Worldwide